- About Us
-
Business Areas
Business Areas
- Our Technology
- Business Performance
-
Customer Support
Customer Support
Our Technology
A company that values the environment
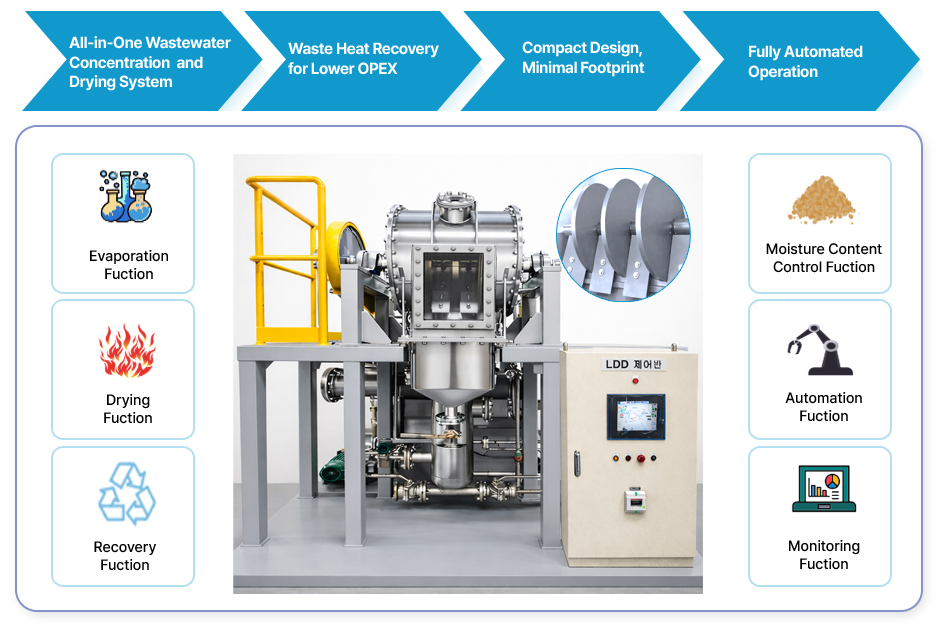
Smart Liquid Disc Drying System
Application field
- 01
- Environmental·Public Sector
- Landfill Leachate
- Incinerator Scrubber Wastewater
- 02
- Secondary Battery
- Secondary Battery High-Salinity Wastewater
- Wash Water & Process Concentrate
- 03
- Semiconductor · Display
- Semiconductor Process Wastewater
- Display Manufacturing Wastewater
- 04
- Metal · Surface Treatment
- Cutting Oil Wastewater
- Heavy Metal Wastewater
- 05
- Food · Pharma · Livestock
- Food & Pharmaceutical Process Wastewater
- Livestock Wastewater
- 06
- System Integration
- Wastewater Reuse Process
- ZLD System
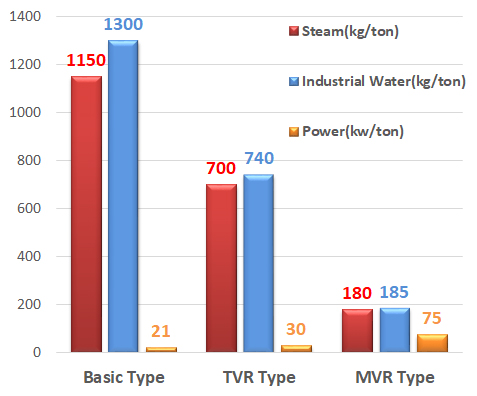
Utility consumption

 Kovec Co., Ltd.
Kovec Co., Ltd.